What does containment mean?
Classification and definition
In the literal sense, this is the "encapsulation" of hazardous substances. The aim is to prevent hazardous substances from escaping from the production process or a product from being contaminated by foreign substances in the environment.
Primary containment
Primary containment refers to all measures that minimise the risk of a substance spreading/infiltrating from or into the production equipment. These include endless liner systems, gloveboxes or double flaps. These solutions ensure that product and operating personnel remain strictly separated from each other throughout the entire process. This not only increases safety, but also ensures sustainable product quality.
Secondary containment
Secondary containment are all measures that reduce the spread/intrusion once the primary containment has been overcome.
These include, for example, cleanrooms, airlocks and pressurisation stages. This level of protection serves as an additional barrier to reliably contain contamination. It makes a significant contribution to ensuring the safety of personnel, product and the environment, even in the event of an incident.
Closed systems
Closed systems in the containment area are machines and systems that are primarily used when hazardous substances are used in accordance with Section 10 GefStoffV.
As an essential basic requirement, there must be no open connection between the environment and the inside of the system. Leakage of substances must be avoided in all cases. This also applies to any cleaning or maintenance. Integrated dedusting devices are therefore also part of the basic equipment of closed systems.
Classification of systems
As not all solids are equally hazardous or sensitive, containment systems are categorised into different "classes" according to their application and area of use. Customers and/or legislators specify product-specific limit values for this. The aim of the classification is to select the appropriate systems and components or the "right" working method for the product used. In the industry, a distinction is usually made between the following classifications and limit values:
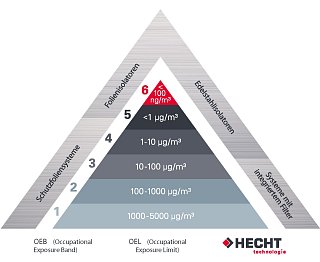
AGW (occupational exposure limit) and OEL (occupational exposure limit) are the average concentration exposure of a substance in the air at the workplace, measured over a defined period of time, at which acute or chronic damage to the health of employees is not to be expected.
Further precise data is required for the exact calculation of this value. ADE (Acceptable Daily Exposure) is the maximum acceptable daily employee exposure. The OEL is calculated from this.
If this data is not available for a substance, a different classification is used. The classification according to OEB (Occupational Exposure Band) only takes into account the toxicity of the pure substance. As a rule of thumb, the more toxic, the higher the classification.
As a competent partner, HECHT is always at your disposal for all questions regarding classification.


Planning of containment systems
Before planning containment production in an active ingredient or pharmaceutical plant, the limit values of the containment to be achieved must be defined. This determines how primary or secondary containment must be designed, or possibly even both. However, the focus should always be on the primary containment and the secondary containment should be installed as an additional measure.
The OEL value or the OEB level determines the containment system to be selected. This is determined internally by the manufacturer on the basis of internal regulations and guidelines and with the help of special measuring devices and laboratory tests.
There are various factors that need to be clarified in advance when planning a containment system:
The products and product quantities used by the customer, as well as the frequency of product changeover, determine whether a mono or multi-purpose system or degree of automation of the system is required. The product properties and flow behaviour must also be assessed. Depending on the substance, explosion protection must also be guaranteed.
System planning is carried out from the inside out and requires extensive knowledge of the processes and process engineering and their critical containment interfaces.
These can be found, for example, in dosing systems, shut-off valves and within the exhaust air systems and filter systems. Above all, all process interfaces and intervention points for operators are places that need to be considered carefully when designing the systems.
Cleaning the systems is of particular importance in order to prevent cross-contamination, product contamination and carry-over. Additional systems that clean according to WIP, CIP or SIP methods are recommended here. These processes must be taken into account during the planning stage; subsequent integration is only possible with immense effort. It goes without saying that the cleaning process must be validatable.
The maintenance and servicing of the containment system must also be considered in conjunction with the cleaning process at the planning stage. Depending on the system, the process system may have to be opened for maintenance work, which should only be carried out after successful cleaning. Wetting surfaces can bind escaping dust and prevent it from escaping.

Requirements for the systems
In principle, a containment system must be designed and operated in such a way that contamination of the environment by dusts or biological agents is prevented. The requirements for the systems are in any case dependent on the product and must be redefined each time. Classifications can be determined using the HECHT pyramid, which is well-known in the industry.
This shows the OEL (Occupational Exposure Limit) or OEB (Occupational Exposure Band). The classification always refers to the toxicity of the products. The maximum number of particles / air volume is then derived from this classification. Once a classification has been created for the product, HECHT offers the appropriate systems.
The systems and devices must now be designed and developed in such a way that they comply with the required limit values. Basic hygiene-compliant design approaches are, for example: dead space-free design, self-contained system or easy accessibility for cleaning.

Qualification of systems
Containment systems should be qualified and validated with regard to their tightness and optimum use in operation.
In order to standardise this, the ISPE published a Good Practice Guide "Assessing the Particulate Containment Performance of Pharmaceutical Equipment" (APCPPE), better known as SMEPAC , in 2004. The guidelines are continuously adapted and revised. In the meantime, this document has established itself as the standard for measurements on containment systems, and the ISPE Containment Manual can also be used as an aid, in which HECHT played a major role in its creation. Containment manual
Various HECHT systems are also measured according to SMEPAC . This means that an OEL (Occupational Exposure Limit) of less than 1 µg/m³ can be achieved with many HECHT systems.

Consideration of the whole
Containment plays a central role in numerous branches of industry. It is particularly relevant in the pharmaceutical industry, where drug and active ingredient manufacturers have to meet the highest standards of purity and safety. Containment is also used in the food sector, for example in the production of high-quality foods, infant formula, nutraceuticals or enzymes. The use of containment solutions has also become established in the chemical industry, particularly in fine chemicals, biotechnology and paint and varnish manufacturers. What all these sectors have in common is that ensuring product safety, occupational safety and process stability is essential. A key challenge here is the consistent avoidance of cross-contamination, as even the smallest particles can cause unintended effects or loss of quality.
It is crucial for success to not only consider isolated individual components, but to always keep the entire process in view. An effective containment concept takes into account all sub-areas and interfaces, from raw material delivery, filling and emptying, in-house transport, dosing and processing through to packaging, storage and finally disposal. Maintenance, cleaning and servicing also play a key role here, as there is an increased risk of substances escaping at these stages in particular.
Containment systems only realise their full benefits if they have a cross-process effect and do not merely solve specific problems. The task is to seamlessly link the various process steps and minimise or completely eliminate the risk of contamination at all critical interfaces as far as possible. This holistic approach is the only way to guarantee an efficient, safe and regulatory-compliant production process.

Containment in production
In manufacturing processes with high containment requirements, there are some factors that need to be considered particularly critically.
Neglecting these can pose a risk to the product, operator and end customer that should not be underestimated. Apart from the relevant FDA regulations or ATEX directives, the points described below must be consistently taken into account and implemented in order not to exceed the limit values defined by the customer in advance, such as AGW or OEL.
First and foremost, it is necessary to include all interfaces of the manufacturing process in order to be able to achieve a certain containment level at all. In addition, the system must be adapted to the respective product with its product-specific properties (e.g. particle size or flow behaviour). When designing the machine components and their connections to exhaust air and filter systems, care must be taken to ensure that the systems are closed. The possibility of residue-free cleaning must also be considered during the development of the system. One of the most critical interfaces is the docking and undocking or the infeed and outfeed of the containers as well as the internal transport to the subsequent process step. The operator in particular has a great deal of responsibility here. The risk of operator errors can only be minimised through intensive training